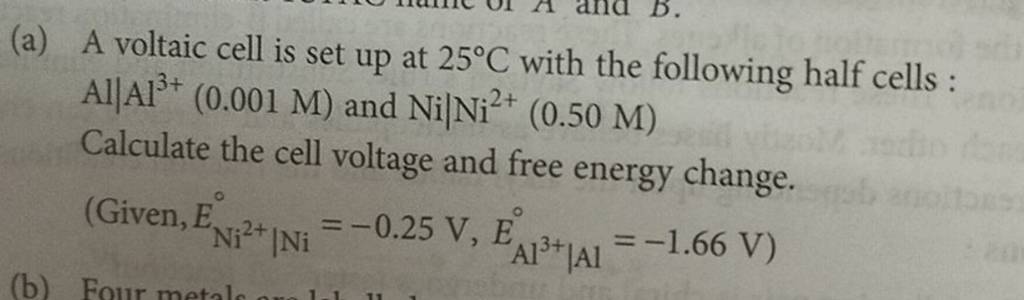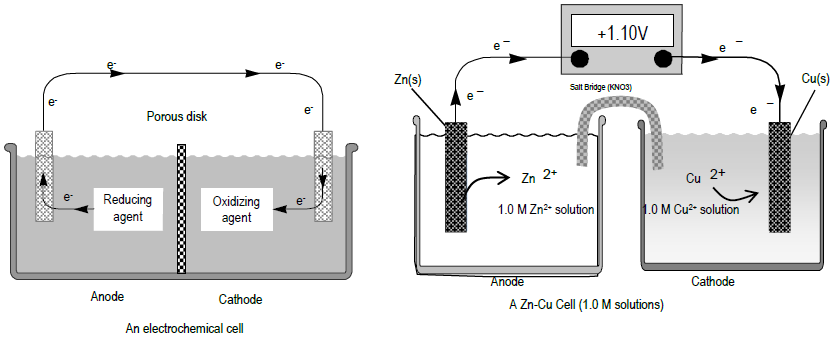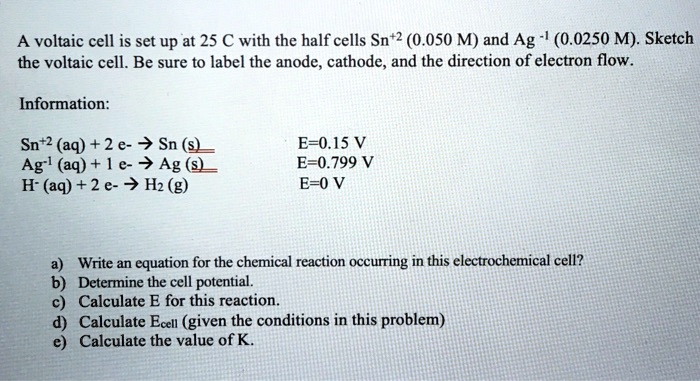
SOLVED: A voltaic cell is set up at 25 C with the half cells Sn+2 (0.050 M) and Ag (0.0250 M). Sketch the voltaic cell. Be sure t0 label the anode, cathode
A voltaic cell is set up at 25°C with the following half cells. Al | Al^3+ (0.0010 M) and Ni^2+ (0.50 M) | Ni - Sarthaks eConnect | Largest Online Education Community
A voltaic cell is set up 25° C with the following half-cells: - Sarthaks eConnect | Largest Online Education Community

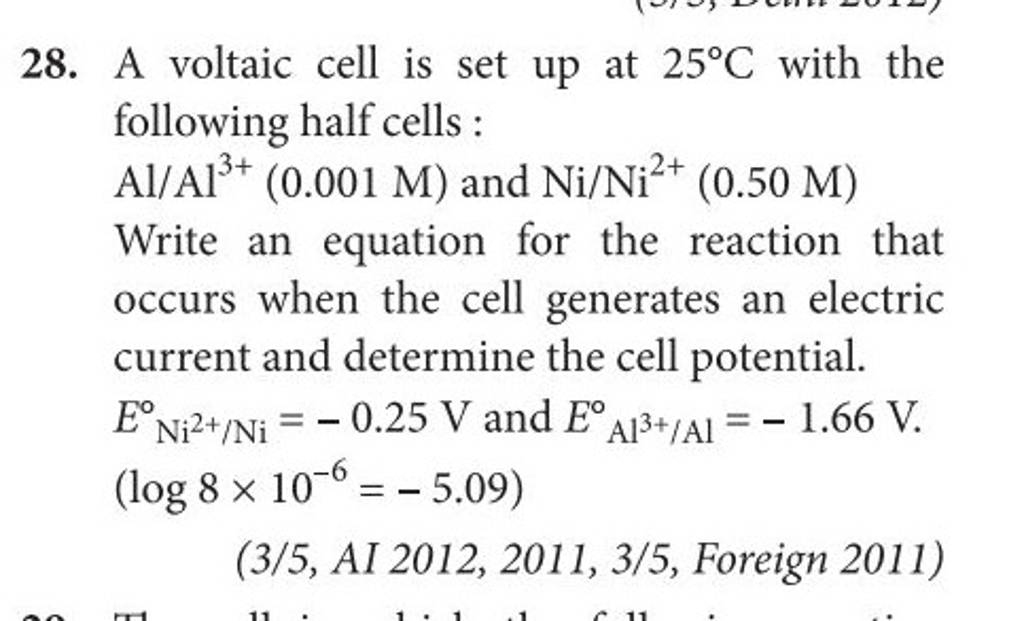
3). A voltaic cell is up 25°C with the following half cells : A1+ (0.001 M) and Ni+2 (0.50 M) Write the cell reaction and calculate the cell potential. (Given : Ex+3/4 = -
A voltaic cell is setup at 25°C with the half cells Ag^+ (0.001 M) Ag and Cu^2+ (0.10 M) Cu. What should be its cell potential ? - Sarthaks eConnect | Largest Online Education Community

A voltaic cell is set-up 25°C with the following half-cells. Ag (0.001 M) | Ag and Cu²+(0.10 M) | Cu What would be the voltage of this cell? [Given, Ecell = 0.46
Please answer this Q1 A voltaic cell is set up at 25 C With following 12 - Chemistry - Electrochemistry - 13799617 | Meritnation.com

A voltaic cell in up 25oC with the following half cells:Al/Al3+(0.001M) and Ni/Ni2+(0.50M)Write an equation the reaction that occurs when the cell generates an electric current and determine the cell potential. ENi2+/Ni=−0.25V

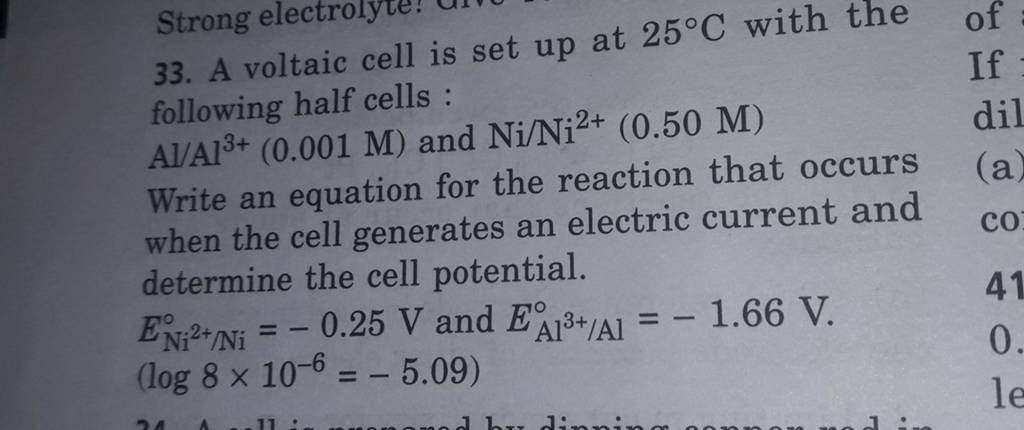


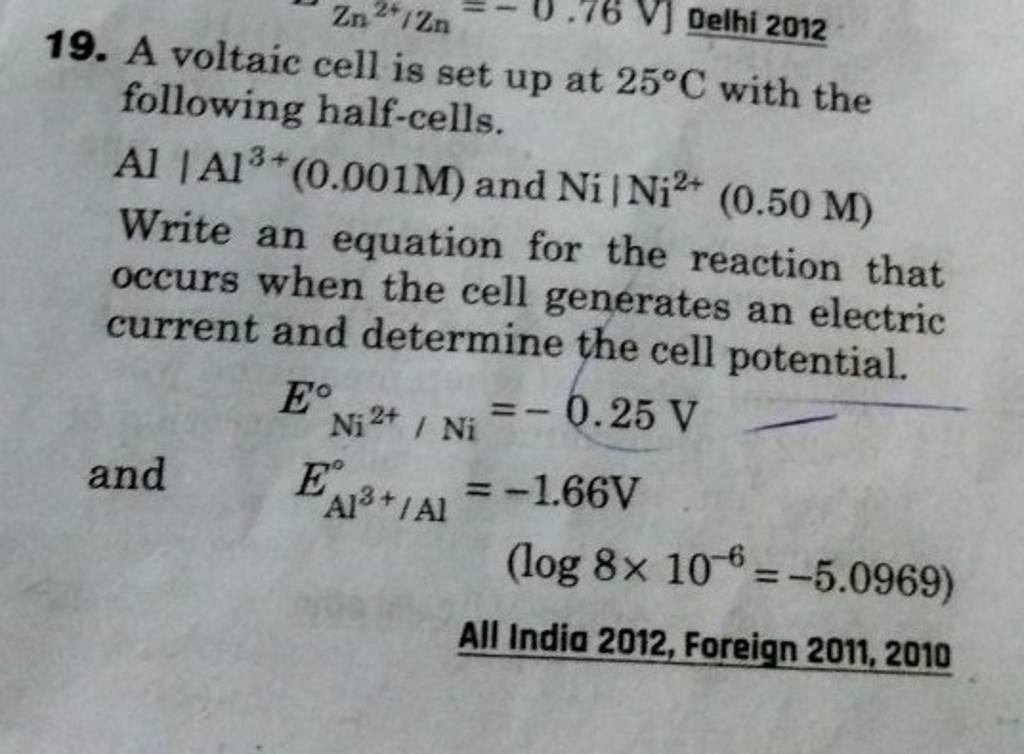
![Kannada] A voltic cell is set up at 25^@C with the half cells Ag^(+) Kannada] A voltic cell is set up at 25^@C with the half cells Ag^(+)](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/7681973.webp)